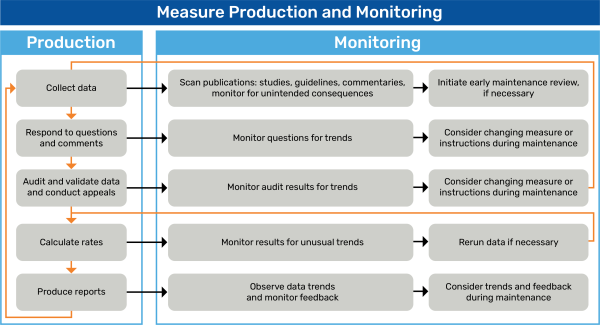
After data collection begins, the measure developer monitors for unintended consequences the measure might have on clinical practice or outcomes. If the measure developer identifies significant unintended consequences in the literature or unusual trends in the data, especially if patient safety is the concern, they must not wait for a scheduled annual or comprehensive review, and an early maintenance review may be necessary.
Respond to Questions About the Measure
The measure developer may be responsible for reviewing interested party feedback and responding promptly, alerting the submitter of receipt and review of the feedback. Interested party feedback may include direct questions, help desk questions, comments about the measure, or the use of the measure in a specific program. Questions may offer ideas for education and outreach.
Monitor and Analyze the Measure Rates and Audit Findings
The measure developer should monitor and analyze measure performance rates and audit findings periodically. At a minimum, developers should perform annual assessments.
- Annual Assessments
Annual assessments monitor and analyze for
- Overall performance trends
- Variations in performance, gaps in care, and extent of improvement
- Analysis of any gaps in quality of care
- Frequency of use of denominator exclusions, numerator exclusions, or denominator exceptions
- Discretionary denominator/numerator exclusion, evaluate carefully for gaming, unintended consequences, and uneven application that could influence comparability
- Patterns of errors in data collection or rate calculation
- Changes in practice that may adversely affect rates Impact of measurement activities on measured entities
- Correlation of the data to either confirm the measure’s efficacy or identify weaknesses in the measure
Ongoing monitoring should continually assess a measure’s progression; any marked departures may be cause for concern. If the business case predicted performance targets, the measure developer should investigate any measure whose performance over time falls short of its target. Measure developers report this information during reevaluation.
Perform Measure Maintenance or Early Maintenance Review, When Appropriate
The measure developer should review each measure at least annually to ensure the codes used to identify the populations (e.g., denominator, numerator, denominator and/or numerator exclusions) are current, and to address other needed minor changes. Communicate all changes to interested parties. Measure Maintenance Reviews describe the standardized processes for the annual update, the triennial full reevaluation, and early maintenance review.
If the CMS consensus-based entity (CBE) has endorsed the measure, the measure developer also reports the results of the maintenance review to the CMS CBE to reevaluate its endorsement at the time of CMS CBE maintenance review.