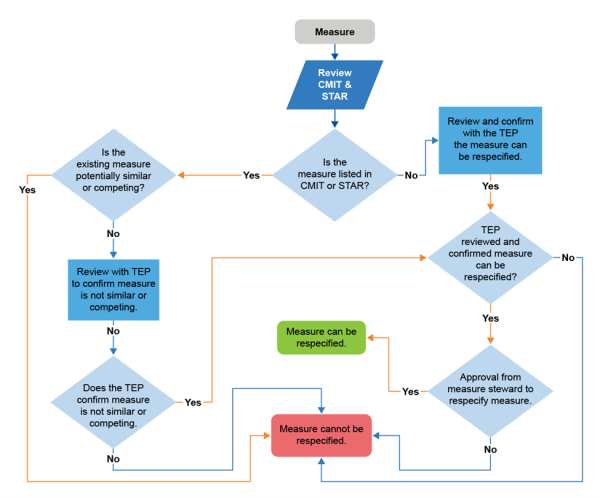
A respecified measure is an existing measure a measure developer changes to fit the current purpose or use, which may mean changing the measure to meet the needs of a different care setting, data source, or population. It may require modifying the numerator, denominator, or adding new building block components to the specifications to fit the new use. For example
- The measure Changes in Skin Integrity Post-Acute Care: Pressure Ulcer/Injury (CMIT Measure ID 121) is an example of respecification to fit the various care settings it's used in such as nursing homes, long-term care hospitals, and inpatient rehabilitation facilities. In this example, the data sources are conceptually similar. When data sources are disparate, such as respecifying from a registry measure to an electronic clinical quality measure (eCQM), there are usually, often greater, challenges in respecification, than if specifying a de novo measure.
The first step in evaluating, via information gathering, whether to respecify a measure is to assess the applicability of the measure focus to the population or care setting of interest or data source:
- Is the focus of the existing measure applicable to the quality goal of the new measure population, setting, or data source?
- Does it meet the importance criterion for the new population or setting?
For example, if the population changes or if the type of data is different, the measure developer creates new specifications and properly evaluates for reliability, validity, and feasibility before determining use in a different setting. There may be a need for empirical analysis to evaluate the appropriateness of the measure for a new purpose. In respecifying a measure to a different setting, the measure developer needs to consider accountability, attribution, and the data source(s) of the new setting. Measures being respecified for use in a different, but similar setting or a different unit of analysis may not need to undergo the same level of comprehensive testing or evaluation compared to a de novo measure. However, when respecifying a measure for use in a new setting, a new population, or with a new data source, the measure developer must evaluate and test the newly respecified measure.
- To assist measure developers in their respecification efforts and before deciding to respecify a measure, the measure developer should consider these questions:
- Are there changes in the relative frequency of critical conditions used in the existing measure specifications when applied to a new setting/population (e.g., when the exclusionary conditions have increased dramatically)?
- Is there a change in the importance of the existing measure in a new setting? For example, an existing measure addressing a highly prevalent condition may not show the same prevalence in a new setting or evidence of large disparities or suboptimal care in the new setting/population.
- Are there changes in the applicability of the existing measure, e.g., the existing measure composite contains preventive care components not appropriate in a new setting such as hospice care?
- Are the data elements required by the existing measure concept available in data source(s) for the respecified measure? This is especially true when respecifying to a digital measure.
- Is it feasible to collect the data elements when changing the data source to an electronic health record (EHR) or another digital format?
- Can the measure developer represent the data elements required in the existing measure in the same terminologies as in the respecified measure?
- Are the data elements valid, e.g., certain codes in the claims from commercial health plans may not be valid or payable under Medicare?
- Is the respecified measure capturing the intended numerator or denominator when applied to a different care setting?
- Are there industry standards (e.g., Health Level Seven International® [HL7®], Interoperability Standards Advisory [ISA], and United States Core Data for Interoperability [USCDI]) the measure must leverage in the respecified version of the measure not included in the existing measure?
- If respecifying a registry measure, are there any non-standard data retrieval, calculation algorithms, or software modules used in a registry or other collection system for the existing measure that require development for the respecified measure?
- Are there clinical workflow, technical, or data flow considerations specific to the respecified measure requiring scrutiny? There will almost certainly be some workflow/data flow impacts when going from a centralized process, e.g., a registry collects data and calculates outcomes, to more of a decentralized EHR implementation-specific data capture and calculation process such as for eCQMs.
- Are there any specialty or setting-specific factors affecting specification and reporting such as for hospital-based specialties, e.g., radiology and pathology, which may use hospital as opposed to outpatient EHR systems and ancillary systems such as laboratory and imaging information systems rather than outpatient EHRs? In such situations, especially where registries are involved, how can the measure logic capture or map the data required in these ancillary systems to the EHR for calculation and reporting?
- What varying or additional procedural, logistical, or timeline requirements exist for the respecified version of the measure? For example, Qualified Registry and Qualified Clinical Data Registry self-nomination submission and timing requirements vary from the pre-rulemaking submission requirements.
- Are there additional formal measure maintenance requirements for the respecified measure, for example, eCQMs require annual updates?
- Are there additional attribution level or program-specific requirements for the respecified measure?
- Considerations for attribution approaches
- If CMS CBE-endorsed, are the changes to the existing measure substantive enough to require resubmission to the CMS CBE for endorsement? The measure developer should discuss endorsement status with the CMS CBE. Measures respecified to eCQMs require resubmission as a new measure. After making any changes to the numerator and denominator statement to fit the specific use, the measure developer needs to create new detailed specifications.
- Will the measure steward be agreeable to the changes in the measure specifications to meet the needs of the current project? If a measure is copyright protected, consider issues (e.g., stewardship, proper referencing of the parent measure, or costs associated with the copyright) relating to the measure’s copyright. In any case, the measure developer should contact the measure steward for permission or clarification.