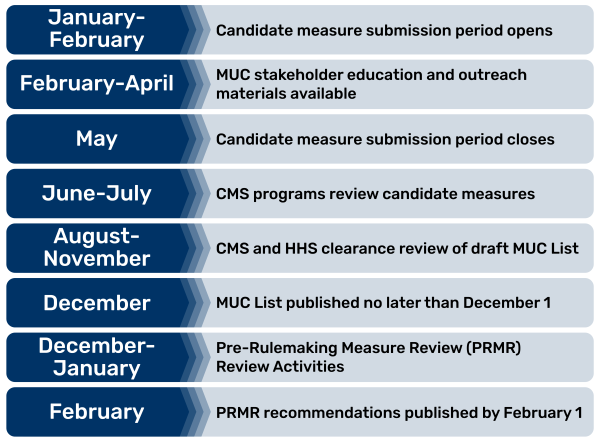
The pre-rulemaking process includes five major steps:
- Each year CMS invites measure developers/stewards to submit candidate measures through the CMS MERIT. The submission period closes on a prescribed date to allow HHS time to review and make their selection of measures to place on the CMS MUC List.
- Annually, no later than December 1, HHS makes publicly available a list of quality and efficiency measures that HHS is considering adopting, through the federal rulemaking process, for use in Medicare program(s).
- Groups of interested parties, convened by the CMS consensus-based entity, provide recommendations to HHS no later than February 1 annually on the quality and efficiency measures under consideration.
- HHS considers the interested parties' input in selecting candidate quality and efficiency measures.
- HHS selects candidate measures and publishes proposed rules in the Federal Register, which allows for public comment and further consideration before a final rule is issued. If the CMS consensus-based entity has not endorsed a candidate measure, then HHS must include a rationale for the use of the measure in the notice.
Click image to enlarge.
Contact for More Information
For more information about the pre-rulemaking process, inquiries about measures submitted for the most recent cycle, or questions on other general measure topics, please email MMSsupport@battelle.org.